Inflammatory conditions such as atopic dermatitis (eczema) and asthma can be debilitating for those who suffer from them. The constant itchiness, redness, and inflammation can have a significant impact on a person’s quality of life. Fortunately, there is now a breakthrough treatment available that can help alleviate these symptoms and improve the overall health and wellbeing of patients: Dupixent.
What is Dupixent?
Dupixent is a prescription medication that contains the active ingredient Dupilumab. It was approved by the U.S. Food and Drug Administration (FDA) in 2017 for the treatment of moderate-to-severe atopic dermatitis in adults. Since then, it has also been approved for use in adolescents with the same condition, as well as for the treatment of moderate-to-severe asthma in adults and adolescents.

How does Dupixent work?
Dupixent works by blocking a protein called interleukin-4 (IL-4) and interleukin-13 (IL-13), which are involved in the inflammatory response that causes the symptoms of atopic dermatitis and asthma. By blocking these proteins, Dupixent helps to reduce inflammation and improve symptoms such as itchiness, redness, and swelling.
Dupixent Mechanism of Action
“DUPIXENT is the only dual inhibitor of IL-4 and IL-13 signaling, inhibiting two key sources of local and systemic type 2 inflammation in asthma”.
What are the benefits of Dupixent?
For those suffering from atopic dermatitis, Dupixent has been shown to significantly improve skin symptoms such as itchiness, redness, and inflammation. In clinical trials, patients treated with Dupixent experienced greater improvements in their symptoms than those treated with a placebo. Dupixent has also been shown to reduce the need for topical corticosteroids, which are commonly used to treat atopic dermatitis but can have side effects such as skin thinning and increased risk of infection.
For those with moderate-to-severe asthma, Dupixent has been shown to improve lung function and reduce the number of asthma exacerbations. In clinical trials, patients treated with Dupixent had a 59% reduction in the rate of severe asthma exacerbations compared to those treated with a placebo. Dupixent has also been shown to improve quality of life for patients with asthma, as well as reduce the need for oral corticosteroids, which can have side effects such as weight gain and increased risk of infection.
Dupixent Indications
- Moderate-to-severe atopic dermatitis (eczema) in patients 6 years of age and older who are not adequately controlled with topical prescription therapies or when those therapies are not advisable.
- Moderate-to-severe asthma in patients aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma.
- Atopic Dermatitis
- Eosinophilic Esophagitis
- Prurigo Nodularis
Dupixent FDA Approval
The U.S. Food and Drug Administration (FDA) has approved Dupixent (dupilumab) for several indications, including:
- Moderate-to-severe atopic dermatitis (eczema) in adults and adolescents aged 12 years and older who are candidates for systemic therapy.
- Moderate-to-severe asthma in patients aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma.
- Chronic rhinosinusitis with nasal polyposis in adults aged 18 years and older who have not adequately responded to other treatments.
Dupixent was granted breakthrough therapy designation by the FDA for the treatment of eosinophilic asthma in 2014, and the FDA granted priority review for the drug for the treatment of moderate-to-severe atopic dermatitis in 2016. The FDA’s approvals of Dupixent were based on clinical studies that demonstrated the drug’s safety and efficacy in these patient populations.
The FDA continues to monitor the safety and effectiveness of Dupixent, as it does with all approved drugs, and healthcare providers are advised to report any adverse events associated with the use of Dupixent to the FDA’s MedWatch program.
What are the side effects of Dupixent?
Like all medications, Dupixent can cause side effects. The most common side effects include injection site reactions (such as redness, swelling, and itching), eye and eyelid inflammation (conjunctivitis and blepharitis), and cold sores. In rare cases, Dupixent can also cause an allergic reaction, which may include swelling of the face, lips, or tongue, hives, and difficulty breathing. If you experience any of these symptoms, you should seek medical attention immediately.
Adverse Reactions of Dupixent
Below are the adverse reactions of Dupixent medication:
| 1. Atopic Dermatitis | 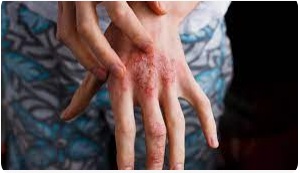 |
| 2. Asthma |  |
| 3. Chronic Rhinosinusitis with Nasal Polyposis |  |
| 4. Eosinophilic Esophagitis | 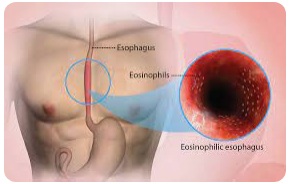 |
| 5. Prurigo Nodularis | 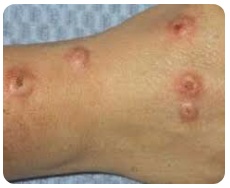 |
Who can take Dupixent?
Dupixent is approved for use in adults and adolescents (aged 12 years and older) with moderate-to-severe atopic dermatitis and moderate-to-severe asthma. However, it may not be suitable for everyone. You should discuss your medical history and any medications you are currently taking with your doctor before starting treatment with Dupixent.
Administering Dupixent
Dupixent (dupilumab) is administered by subcutaneous injection (under the skin). Here are the general steps for administering Dupixent:
- Check the Dupixent carton to make sure that the expiration date has not passed and that the medication has been stored at the proper temperature (2°C to 8°C or 36°F to 46°F).
- Gather all necessary supplies, including the Dupixent prefilled syringe or autoinjector, alcohol swabs, cotton balls or gauze, and a sharps disposal container.
- Choose an injection site on the thigh or abdomen (except for the 2-inch area right around the belly button) that is at least 2 inches away from any other injection site and clean the area with an alcohol swab.
- Remove the cap from the Dupixent syringe or autoinjector and wipe the injection site with an alcohol swab.
- Pinch a fold of skin at the injection site and insert the needle at a 45-degree angle for the syringe or 90-degree angle for the autoinjector.
- Inject the full amount of Dupixent as prescribed by your healthcare provider and remove the needle.
- Apply pressure to the injection site with a cotton ball or gauze and dispose of the used needle and syringe in a sharps disposal container.
- If using the Dupixent Room Temperature Kit, make sure to keep the medication at room temperature and use it within 14 days.
It is important to follow your healthcare provider’s instructions on how to administer Dupixent and to report any injection site reactions or other side effects to your healthcare provider.
Dupixent USPI
The USPI (United States Prescribing Information) for Dupixent contains detailed information about the medication, including its approved uses, dosage and administration, contraindications, warnings and precautions, adverse reactions, and drug interactions. The USPI is an important resource for healthcare providers and patients to review before starting Dupixent treatment.
Some of the key information contained in the Dupixent USPI (here) includes:
- Approved uses: Dupixent is approved for the treatment of moderate-to-severe atopic dermatitis (eczema) in patients 6 years of age and older who are not adequately controlled with topical prescription therapies or when those therapies are not advisable. It is also approved for the treatment of moderate-to-severe asthma in patients aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma.
- Dosage and administration: The recommended dose of Dupixent for atopic dermatitis is based on body weight and ranges from 300 mg to 600 mg every two weeks, depending on the patient’s weight. The recommended dose for asthma is 200 mg every two weeks. Dupixent is administered by subcutaneous injection.
- Contraindications: Dupixent is contraindicated in patients with a history of hypersensitivity to dupilumab or any of its excipients.
- Warnings and precautions: Dupixent may increase the risk of infection, including serious infections such as pneumonia and cellulitis. It may also increase the risk of hypersensitivity reactions, including anaphylaxis. Dupixent should not be used to treat acute bronchospasm or status asthmaticus. Dupixent should be used with caution in patients with pre-existing eosinophilic conditions or with a history of parasitic infections.
- Adverse reactions: The most common adverse reactions associated with Dupixent treatment include injection site reactions, eye and eyelid inflammation, and cold sores. Dupixent may also increase the risk of conjunctivitis, herpes simplex infections, and hypersensitivity reactions.
- Drug interactions: There are no known drug interactions with Dupixent, but caution should be used when administering live vaccines to patients receiving Dupixent.
This is just a summary of the information contained in the Dupixent USPI, and it is recommended to review the full USPI for complete information on the medication.
Dupixent EMA
The European Medicines Agency (EMA) has approved Dupixent (dupilumab) for the following indications:
- Moderate-to-severe atopic dermatitis (eczema) in adults and adolescents aged 12 years and older who are candidates for systemic therapy.
- Severe asthma with type 2 inflammation characterized by raised blood eosinophils and/or raised fractional exhaled nitric oxide (FeNO) in adults and adolescents aged 12 years and older who are inadequately controlled with high-dose inhaled corticosteroids plus another medicinal product for maintenance treatment.
In addition, the EMA has also approved Dupixent for the treatment of:
- Severe chronic rhinosinusitis with nasal polyposis (CRSwNP) in adults aged 18 years and older who are inadequately controlled with intranasal corticosteroids and/or after surgery.
The EMA evaluates the safety, efficacy, and quality of medicinal products in the European Union (EU) to ensure that they meet the required standards for approval and use. The EMA’s approval of Dupixent for these indications means that it is authorized for use in the EU and can be prescribed by healthcare providers to eligible patients.
Dupixent Blue Cross Blue Shield
Dupixent is covered by most Blue Cross Blue Shield insurance plans, but the specific coverage and cost-sharing requirements may vary depending on your individual policy. Some insurance plans may require prior authorization or have specific coverage criteria that must be met before they will cover Dupixent. It is recommended to check with your insurance provider or healthcare provider to confirm coverage and copay amounts for Dupixent under your Blue Cross Blue Shield plan. Additionally, some patients may be eligible for financial assistance through programs offered by the manufacturer or other organizations. It is important to explore all available options for obtaining Dupixent at an affordable cost.
Regeneron Sanofi Dupixent
Regeneron and Sanofi are the companies that developed Dupixent (dupilumab) and co-market the medication in the United States. Regeneron is a biotechnology company that specializes in developing novel therapies for serious medical conditions, while Sanofi is a global pharmaceutical company that focuses on researching, developing, and marketing healthcare products.
The development of Dupixent was a joint effort between Regeneron and Sanofi, with Regeneron responsible for the research and development of the drug, and Sanofi responsible for the clinical development, regulatory affairs, and commercialization of the drug. The two companies have collaborated on several other successful products, including Kevzara (sarilumab) and Praluent (alirocumab), which are both used to treat cardiovascular disease.
Dupixent has been a significant success for Regeneron and Sanofi, with the medication receiving approvals for multiple indications, including moderate-to-severe atopic dermatitis (eczema), moderate-to-severe asthma, and severe chronic rhinosinusitis with nasal polyposis (CRSwNP). The collaboration between Regeneron and Sanofi has helped to bring this important therapy to patients in need and highlights the potential benefits of partnerships between biotechnology and pharmaceutical companies in developing novel treatments for serious medical conditions.
Dupixent Out of Fridge Storage
Dupixent should be stored in a refrigerator between 2°C to 8°C (36°F to 46°F) until the time of use. However, if needed, Dupixent can be kept at room temperature between 15°C to 25°C (59°F to 77°F) for a maximum of 14 days. This is known as the Dupixent Room Temperature Kit. The Room Temperature Kit is designed for patients who need to travel with Dupixent or for those who may not have access to a refrigerator. It is important to note that once Dupixent is removed from the refrigerator and brought to room temperature, it must be used within 14 days or discarded. If Dupixent has been left at room temperature for more than 14 days, it should be discarded and not used. It is recommended to speak with your healthcare provider or pharmacist for guidance on proper storage and use of Dupixent.
Stopping Dupixent
If you are taking Dupixent (dupilumab) and are considering stopping treatment, it is important to first discuss your options with your healthcare provider. Dupixent is typically prescribed for the long-term management of certain chronic conditions, such as atopic dermatitis or asthma, and stopping treatment abruptly can potentially lead to a worsening of symptoms.
Your healthcare provider may recommend a gradual tapering of Dupixent over time to minimize the risk of rebound effects or worsening of symptoms. It is important to follow your healthcare provider’s instructions for tapering off Dupixent, as abruptly stopping the medication can lead to an increased risk of adverse events.
If you experience any new or worsening symptoms after stopping Dupixent, it is important to contact your healthcare provider as soon as possible. Your healthcare provider may recommend restarting treatment or adjusting your treatment plan to help manage your symptoms.
Overall, the decision to commence or stop Dupixent should be made in consultation with your healthcare provider and based on your individual medical needs and treatment goals.
Frequently asked questions
1. Does Dupixent cure eczema or asthma?
Dupixent is not a cure for eczema or asthma, but it can help manage symptoms and reduce the frequency and severity of flare-ups. It works by blocking specific proteins that play a key role in the inflammatory process that causes eczema and asthma. While Dupixent can provide significant relief for those who suffer from these conditions, it is not a permanent solution and the symptoms may return if the medication is discontinued. Therefore, it is important to continue to work closely with your healthcare provider to manage your condition effectively.
2. Is Dupixent covered by insurance?
Dupixent is covered by most insurance plans, including Medicare and Medicaid. However, the specific coverage and cost-sharing requirements may vary depending on your insurance plan and individual policy. Some insurance plans may require prior authorization or have specific coverage criteria that must be met before they will cover Dupixent. Additionally, some patients may be eligible for financial assistance through programs offered by the manufacturer or other organizations. It is recommended to check with your insurance provider or healthcare provider to confirm coverage and copay amounts, and to explore any financial assistance options that may be available to you.
3. How much does Dupixent cost?
The cost of Dupixent varies depending on several factors, such as the dosage, frequency of administration, and insurance coverage. Without insurance, the average cost of Dupixent can range from $3,000 to $6,000 per month. However, many patients may be able to obtain Dupixent for a lower cost through insurance coverage or financial assistance programs. The manufacturer of Dupixent offers a patient assistance program to help eligible patients obtain the medication at a reduced cost or for free, depending on their financial situation. Additionally, some patients may be eligible for copay assistance programs through their insurance provider or other organizations. It is recommended to check with your insurance provider or healthcare provider to explore all available options for obtaining Dupixent at an affordable cost.
4. What is the annual cost of Dupixent?
The annual cost of Dupixent can vary depending on several factors, including the dosage, frequency of administration, and insurance coverage. Without insurance, the average cost of Dupixent can range from $36,000 to $72,000 per year, based on the monthly cost range of $3,000 to $6,000. However, many patients may be able to obtain Dupixent for a lower cost through insurance coverage or financial assistance programs. The actual out-of-pocket cost for Dupixent can vary widely based on the individual’s insurance coverage and the specific policy, as well as any available copay or financial assistance programs. It is recommended to check with your insurance provider or healthcare provider to obtain a more accurate estimate of the annual cost of Dupixent based on your individual situation.
5. What is the cash price of Dupixent?
The cash price of Dupixent, meaning the price without insurance coverage, can vary depending on the dosage, frequency of administration, and the pharmacy where it is purchased. Without insurance, the average cost of Dupixent can range from $3,000 to $6,000 per month. Therefore, the cash price for a year’s worth of treatment with Dupixent can range from $36,000 to $72,000. However, some pharmacies may offer discounts or promotions that can lower the cash price. It is important to note that most patients will have some form of insurance coverage, and the actual out-of-pocket cost for Dupixent can vary widely based on the individual’s insurance coverage and the specific policy, as well as any available copay or financial assistance programs. It is recommended to check with your insurance provider or healthcare provider to obtain a more accurate estimate of the cost of Dupixent based on your individual situation.
6. What is the retail price of Dupixent?
The retail price of Dupixent, meaning the price listed by the manufacturer, can vary depending on several factors such as the dosage, frequency of administration, and location. The retail price is typically higher than the negotiated price with insurance providers, so the actual cost to patients may be lower than the retail price. The manufacturer of Dupixent does not publicly disclose the retail price, but it is estimated to be around $3,000 to $6,000 per month without insurance coverage. However, it is important to note that the actual out-of-pocket cost for Dupixent can vary widely based on the individual’s insurance coverage and the specific policy, as well as any available copay or financial assistance programs. It is recommended to check with your insurance provider or healthcare provider to obtain a more accurate estimate of the cost of Dupixent based on your individual situation.
7. Is DUPIXENT a steroid?
No, Dupixent (dupilumab) is not a steroid. It is a monoclonal antibody that works by blocking specific proteins in the immune system that contribute to the development of certain chronic skin and respiratory conditions, such as atopic dermatitis and asthma.
In contrast, steroids are a class of drugs that are commonly used to reduce inflammation in the body. Steroids can be effective in treating a wide range of medical conditions, but they can also have side effects, such as weight gain, mood changes, and increased risk of infection.
While both Dupixent and steroids can be used to treat similar conditions, they work through different mechanisms and have different potential side effects. Your healthcare provider can help determine the best treatment option for your individual needs and medical history.
8. What is the downside of DUPIXENT?
Like any medication, Dupixent (dupilumab) may have potential downsides or side effects. It is important to discuss these risks with your healthcare provider before starting treatment with Dupixent.
Some potential downsides or side effects of Dupixent may include:
- Injection site reactions: Some patients may experience redness, swelling, or itching at the injection site.
- Conjunctivitis: Dupixent may increase the risk of developing conjunctivitis (pink eye) or other eye infections.
- Allergic reactions: Some patients may experience allergic reactions to Dupixent, including rash, hives, or difficulty breathing.
- Increased risk of infection: Dupixent may increase the risk of infections, particularly those caused by bacteria, viruses, or fungi.
- Elevated eosinophil levels: Dupixent may increase eosinophil levels, which can lead to inflammation and other symptoms.
It is important to note that not all patients who take Dupixent will experience these side effects, and many patients find that the benefits of treatment outweigh any potential risks. Your healthcare provider can help determine if Dupixent is a safe and appropriate treatment option for your individual needs and medical history.


![Best Drug Rehab Centers in the US 2023 [Top-Rated] Best Drug Rehab Centers](https://www.medicosrepublic.com/wp-content/uploads/2023/02/Best-Drug-Rehab-Centers-in-the-US-218x150.jpg)

![Therapy Outcome Measures for Rehabilitation Professionals PDF Free Download [Direct Link] Therapy Outcome Measures for Rehabilitation Professionals PDF](https://www.medicosrepublic.com/wp-content/uploads/2024/03/Therapy-Outcome-Measures-for-Rehabilitation-Professionals-PDF-Free-Download-1-150x150.jpg)
![Braunwald’s Heart Disease Review and Assessment 10th Edition PDF Free Download [Direct Link] Braunwald’s Heart Disease Review and Assessment 10th Edition PDF](https://www.medicosrepublic.com/wp-content/uploads/2018/07/Braunwald’s-Heart-Disease-Review-and-Assessment-10th-Edition-PDF-Free-Download-150x150.jpg)
![1001 Tips For Orthodontics And Its Secrets PDF Free Download [Direct Link] 1001 Tips For Orthodontics And Its Secrets PDF](https://www.medicosrepublic.com/wp-content/uploads/2023/07/1001-Tips-For-Orthodontics-And-Its-Secrets-PDF-150x150.jpg)
![Medical Coding Exam Prep ICD-10 PDF Free Download [Direct Link] Medical Coding Exam Prep ICD-10 PDF](https://www.medicosrepublic.com/wp-content/uploads/2024/04/Medical-Coding-Exam-Prep-ICD-10-PDF-Free-Download-1-150x150.jpg)
![Pocketbook of Clinical IR: A Concise Guide to Interventional Radiology PDF Free Download [Direct Link]](https://www.medicosrepublic.com/wp-content/uploads/2023/02/Pocketbook-of-Clinical-IR-A-Concise-Guide-to-Interventional-Radiology-PDF-Free-Download-150x150.jpg)
![Medical-Surgical Nursing Made Incredibly Easy! PDF Free Download [Direct Link] Medical-Surgical Nursing Made Incredibly Easy PDF](https://www.medicosrepublic.com/wp-content/uploads/2018/03/Medical-Surgical-Nursing-Made-Incredibly-Easy-PDF-Free-Download-150x150.jpg)
![Paediatrics at a Glance 4th Edition PDF Free Download [Direct Link] Paediatrics at a Glance 4th Edition PDF](https://www.medicosrepublic.com/wp-content/uploads/2023/07/Paediatrics-at-a-Glance-4th-Edition-PDF-Free-Download-1-150x150.jpg)
![Guide to Evidence-Based Physical Therapist Practice 4th Edition PDF Free Download [Direct Link]](https://www.medicosrepublic.com/wp-content/uploads/2022/06/Guide-to-Evidence-Based-Physical-Therapist-Practice-4th-Edition-696x412-1-150x150.jpg)
![The Equine Hospital Manual 1st Edition PDF Free Download [Direct Link]](https://www.medicosrepublic.com/wp-content/uploads/2022/06/The-Equine-Hospital-Manual-696x365-1-150x150.jpg)
![Neurology and Clinical Neuroanatomy on the Move (Medicine on the Move) 1st Edition PDF Free Download [Direct Link] Neurology and Clinical Neuroanatomy on the Move (Medicine on the Move) 1st Edition PDF](https://www.medicosrepublic.com/wp-content/uploads/2022/10/Neurology-and-Clinical-Neuroanatomy-on-the-Move-Medicine-on-the-Move-1st-Edition-PDF-Free-Download-150x150.jpg)
![Color Atlas of Veterinary Histology 3rd Edition PDF Free Download [Direct Link] Color Atlas of Veterinary Histology 3rd Edition PDF](https://www.medicosrepublic.com/wp-content/uploads/2024/07/Color-Atlas-of-Veterinary-Histology-3rd-Edition-PDF-Free-Download-100x70.jpg)
![Color Textbook of Histology 3rd Edition PDF Free Download [Direct Link] Color Textbook of Histology 3rd Edition PDF](https://www.medicosrepublic.com/wp-content/uploads/2024/07/Color-Textbook-of-Histology-3rd-Edition-PDF-Free-Download-100x70.jpg)
![Doctor in Training Solid Anatomy Collection Free Download [Direct Link] Doctor in Training Solid Anatomy Collection](https://www.medicosrepublic.com/wp-content/uploads/2024/07/Doctor-in-Training-Solid-Anatomy-Collection-100x70.jpg)
![Willard and Spackman’s Occupational Therapy 11th Edition PDF Free Download [Direct Link] Willard and Spackman's Occupational Therapy 11th Edition](https://www.medicosrepublic.com/wp-content/uploads/2024/07/Willard-and-Spackmans-Occupational-Therapy-11th-Edition-PDF-Free-Download-1-100x70.jpg)